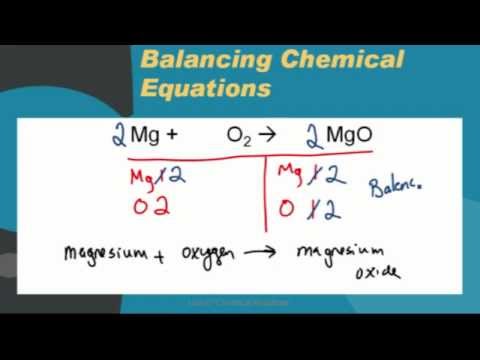
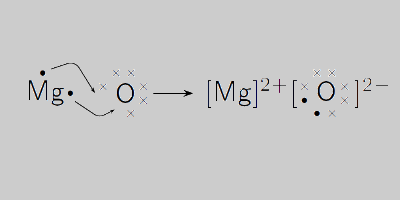Write the equation for the combination of magnesium and oxygen to form magnesium oxide, MgO. | Homework.Study.com

Explain the formation of magnesium oxide from magnesium and oxygen? Analyze the electron dot diagram and complete the table.

CHEMICAL EQUATIONS. Chemical equations You are expected to know these names and formula: NameFormulaNameFormula WaterH2OH2OHydrchloric Acid HCl Carbon. - ppt download
A 3.250g sample of magnesium is burned in a container of 12.500g oxygen. What mass of oxygen gas remains unreacted after the magnesium has been completely consumed to form magnesium oxide as

Metal carbonates are known to undergo thermal decomposition, producing the metal oxide and releasing carbon dioxide. The process is described by the following generic equation, in which M represents an unknown divalent

Question Video: Identifying the Oxidized Specie in the Reaction of Magnesium Oxide with Hydrogen | Nagwa